In vivo cells obtain simultaneous alerts from a number of extracellular ligands and should combine and interpret them to reply appropriately.
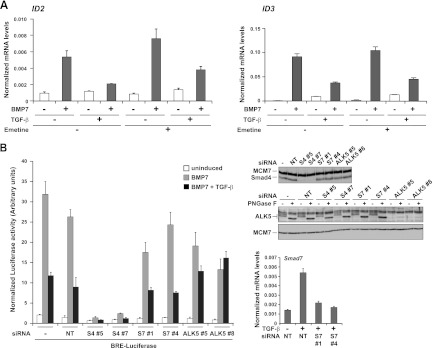
Here we examine the interaction between pathways downstream of two reworking growth factor β (TGF-β) superfamily members, bone morphogenetic protein (BMP) and TGF-β. We present that in a number of cell strains, TGF-β potently inhibits BMP-induced transcription on the stage of each BMP-responsive reporter genes and endogenous BMP goal genes.
This inhibitory impact requires the TGF-β kind I receptor ALK5 and is impartial of recent protein synthesis. Strikingly, we present that Smad3 is required for TGF-β’s inhibitory results, whereas Smad2 shouldn’t be. We go on to exhibit that TGF-β induces the formation of complexes comprising phosphorylated Smad1/5 and Smad3, which bind to BMP-responsive parts in vitro and in vivo and mediate TGF-β-induced transcriptional repression.
Furthermore, lack of Smad3 confers on TGF-β the power to induce transcription through BMP-responsive parts. Our outcomes subsequently recommend that not solely is Smad3 vital for mediating TGF-β’s inhibitory results on BMP signaling nevertheless it additionally performs a essential function in proscribing the transcriptional output in response to TGF-β.
Ectopic bone morphogenetic proteins 5 and four within the hen forebrain result in cyclopia and holoprosencephaly.
Proper dorsal-ventral patterning within the growing central nervous system requires alerts from each the dorsal and ventral parts of the neural tube. Data from a number of research have demonstrated that bone morphogenetic proteins (BMPs) and Sonic hedgehog protein are secreted components that regulate dorsal and ventral specification, respectively, inside the caudal neural tube.
In the growing rostral central nervous system Sonic hedgehog protein additionally participates in ventral regionalization; nevertheless, the roles of BMPs within the growing mind are much less clear. We hypothesized that BMPs additionally play a task in dorsal specification of the vertebrate forebrain. To check our speculation we implanted beads soaked in recombinant BMP5 or BMP4 into the neural tube of the hen forebrain. Experimental embryos confirmed a lack of the basal telencephalon that resulted in holoprosencephaly (a single cerebral hemisphere), cyclopia (a single midline eye), and lack of ventral midline constructions.
In situ hybridization utilizing a panel of probes to genes expressed within the dorsal and ventral forebrain revealed the lack of ventral markers with the upkeep of dorsal markers. Furthermore, we discovered that the lack of the basal telencephalon was the results of extreme cell dying and never a change in cell fates. These information present proof that BMP signaling participates in dorsal-ventral patterning of the growing mind in vivo, and disturbances in dorsal-ventral signaling end in particular malformations of the forebrain.
